Abstract
Background: Pyruvate kinase (PK) deficiency is a rare hemolytic anemia caused by mutations in the PKLR gene. PK deficiency and its management are associated with a wide spectrum of complications. Data indicate there is a high disease burden in pediatric patients (pts) with this condition; the full breadth of clinical presentations and complications has yet to be fully characterized.
Aim: To further describe the comorbidities and complications experienced by pediatric pts with PK deficiency in the Peak Registry (NCT03481738).
Methods: The Peak Registry is an ongoing, global, retrospective, and prospective observational study of pts with a genetically confirmed diagnosis of PK deficiency. This analysis included pediatric pts (<18 years [yrs]) with available age and classifiable PKLR genotype data as of 01Dec2021. Pts were grouped into cohorts by age at enrollment and genotype (missense/missense [M/M]; missense/non-missense [M/NM]; non-missense/non-missense [NM/NM]). NM mutations include nonsense, frameshift, and splicing variants. Demographic data, laboratory values, and medical history inclusive of comorbidities and complications were described for all pts with available data. Continuous variables were summarized using mean, SD, median, and range. Categorical variables were summarized by number and percent of pts within a category.
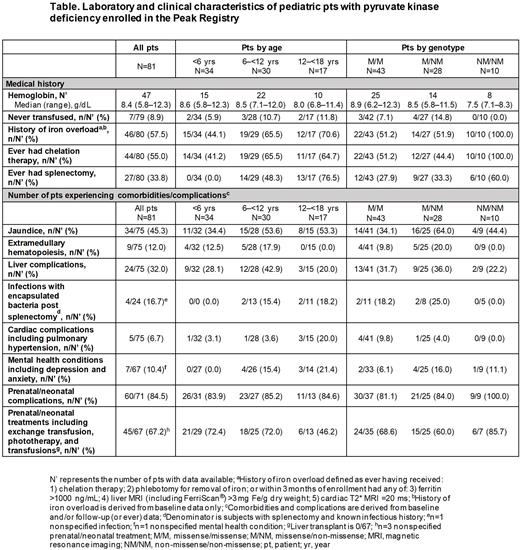
Results: Among 81 pediatric pts, mean age at enrollment was 7.0 yrs (SD: 4.7) and there were 34 (42%) pts aged <6 yrs, 30 (37%) pts aged 6-<12 yrs, and 17 (21%) pts aged 12-<18 yrs. Most pts had ≥1 missense mutation (43 [53%] M/M, 28 [35%] M/NM, and 10 [12%] NM/NM). At enrollment, median hemoglobin in the M/M, M/NM, and NM/NM cohorts was 8.9 g/dL (range: 6.2-12.3), 8.5 g/dL (5.8-11.5), and 7.5 g/dL (7.1-8.3), respectively (Table).
The most common physical exam findings as neonates were neonatal jaundice (69%), hepatomegaly (20%), and splenomegaly (33%). Prenatal/neonatal complications occurred in most pts (85%), including preterm delivery (14%), pulmonary hypertension (7%), hepatopathy/hepatic failure (7%), skin extramedullary hematopoiesis (4%), coronary artery disease (3%), in utero growth retardation (3%), and hydrops fetalis (1%). Prenatal/neonatal management was required in 67% of pts, including phototherapy (52%) and exchange transfusion (24%). Abnormal lab findings in neonates were common: anemia (59%), thrombocytopenia (19%), and hyperferritinemia (7%).
The majority of pts (91%) had received ≥1 transfusion prior to or at enrollment. In the 12 months prior to enrollment, 23/70 (33%) had received regular transfusions (≥6 transfusions in that period) with younger pts more often receiving regular transfusions (16/31 [52%] pts aged <6 yrs, 5/27 [19%] pts aged 6-<12 yrs, and 2/12 [17%] pts aged 12-<18 yrs). Only 7/79 (9%) pts had never received a transfusion (3 M/M and 4 M/NM). Most pts had a history of iron overload, regardless of age (<6 yrs: 44%; 6-<12 yrs: 66%; 12-<18 yrs: 71%) or genotype (M/M: 51%; M/NM: 52%; NM/NM: 100%). Overall, 44/80 (55%) pts had received chelation therapy, including 41% of pts <6 yrs. Specifically, 51%, 44%, and 100% of pts with M/M, M/NM, and NM/NM genotypes, respectively, had received chelation.
Twenty-seven of 80 (34%) pts had undergone splenectomy. Prevalence increased with age (<6 yrs: 0%; 6-<12 yrs: 48%; 12-<18 yrs: 77%) and varied by genotype (M/M: 28%; M/NM: 33%; NM/NM: 60%). Post-splenectomy infections with encapsulated bacteria were common (17%).
Liver complications, including fatty liver, cirrhosis, and hepatomegaly, occurred in 32% of pts and were common across age and genotype subgroups. Other notable complications were biliary events (21%), extramedullary hematopoiesis (12%), and cardiac complications (7%) such as pulmonary hypertension (4%). Overall, 10% of pts reported mental health issues including depression and anxiety.
Conclusions: Pediatric pts with PK deficiency experience a wide range of comorbidities and complications, even at an early age and regardless of genotype. Neonates have varied presentations and complications (eg, hyperferritinemia, thrombocytopenia, pulmonary hypertension) that may be difficult to recognize as PK deficiency, potentially leading to initial misdiagnosis and unnecessary treatments. Clinicians should consider PK deficiency in these cases and be aware of all its early manifestations to ensure pts receive appropriate diagnosis, monitoring, and management.
Disclosures
Grace:Sanofi: Consultancy; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Agios Pharmaceuticals: Consultancy, Research Funding. Glenthøj:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacosmos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Celgene: Consultancy; Saniona: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lander:Agios Pharmaceuticals, Inc.: Other: PK Deficiency Patient Advocacy Advisory Council patient representative. van Beers:Novartis: Research Funding; Pfizer: Research Funding; RR Mechatronics: Research Funding; Sobi: Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Consultancy; Sanofi: Consultancy. Bianchi:Agios Pharmaceuticals, Inc.: Other: Scientific advisor. Williams:Agios Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Yan:Agios Pharmaceuticals, Inc.: Current Employment, Other: Shareholder. McGee:Agios Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Glader:Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal